Icosabutate latest phase 2b interim data show significant decreases in NASH and fibrosis biomarkers independent of fibrosis stage and disease severity
Results presented at the AASLD Liver Meeting Digital Experience™ 2021
- Significant decreases in key markers of inflammation and fibrogenesis were comparable or of greater magnitude in patients with stage 2/3 fibrosis vs the overall population after 16 weeks in the ICONA trial
- Greatest reductions in ALT, PRO-C3 and hsCRP seen in patients with highest baseline levels of these markers of disease severity
- Enrollment completed for ICONA trial in patients with biopsy-confirmed nonalcoholic steatohepatitis, with top-line 52-week histology results expected in Q1-2023
Naarden, The Netherlands, 16 November 2021 – NorthSea Therapeutics B.V., (‘NST’) a biotech company developing novel and innovative strategies for the treatment of NASH and other metabolic diseases, today announces the presentation of new interim data from the Phase 2b ICONA trial during The Liver Meeting Digital Experience™ 2021 (TLMdX) of the American Association for the Study of Liver Diseases (AASLD), which was held online from November 12-15.
These latest data are a post-hoc analysis of the prespecified interim analysis of the Phase 2b ICONA trial in biopsy confirmed NASH patients investigating the treatment response in patients with more severe disease at baseline.
The ICONA study (ICOsabutate in NASH) is an ongoing 52-week, multicenter, placebo-controlled, phase 2b study which enrolled 280 subjects with biopsy confirmed NASH with stage 1-3 fibrosis, NAS ≥ 4 (1 point in each component) and ≥10% liver fat content by MRI-PDFF.
A prespecified interim analysis was performed evaluating multiple non-invasive biomarkers relevant for NASH and fibrosis after 90 participants had been treated through to Week 16. These initial results, presented at the EASL International Liver Congress in early 2021 by Dr. Stephen Harrison, showed a robust treatment effect of icosabutate therapy alongside a favourable safety and tolerability profile.
The study is being conducted at 50 sites across the United States and is now fully enrolled as of 15 November. The top-line 52 weeks results are expected in the first quarter of 2023.
The current poster presentation entitled “Icosabutate, a Novel Oral Free Fatty Acid 4 (FFAR4) Agonist, Significantly Decreases Biomarkers of NASH and Fibrosis Independent of Disease Severity” will be presented by Dr. Naim Alkhouri, VP of Academic Affairs and Director of the Fatty Liver Program at Arizona Liver Health. “These data give further important insights into the rapid and potent biologic activity of icosabutate across multiple pathways relevant to NASH and fibrosis, several of which have been shown to be predictive of histologic response,” stated Dr. Alkhouri.
This post-hoc analysis shows patients with stages 2/3 fibrosis have rapid and sustained significant decreases in all biomarkers at 600 mg and the majority at 300 mg and were comparable or greater than the overall population of stages 1/2/3 fibrosis.
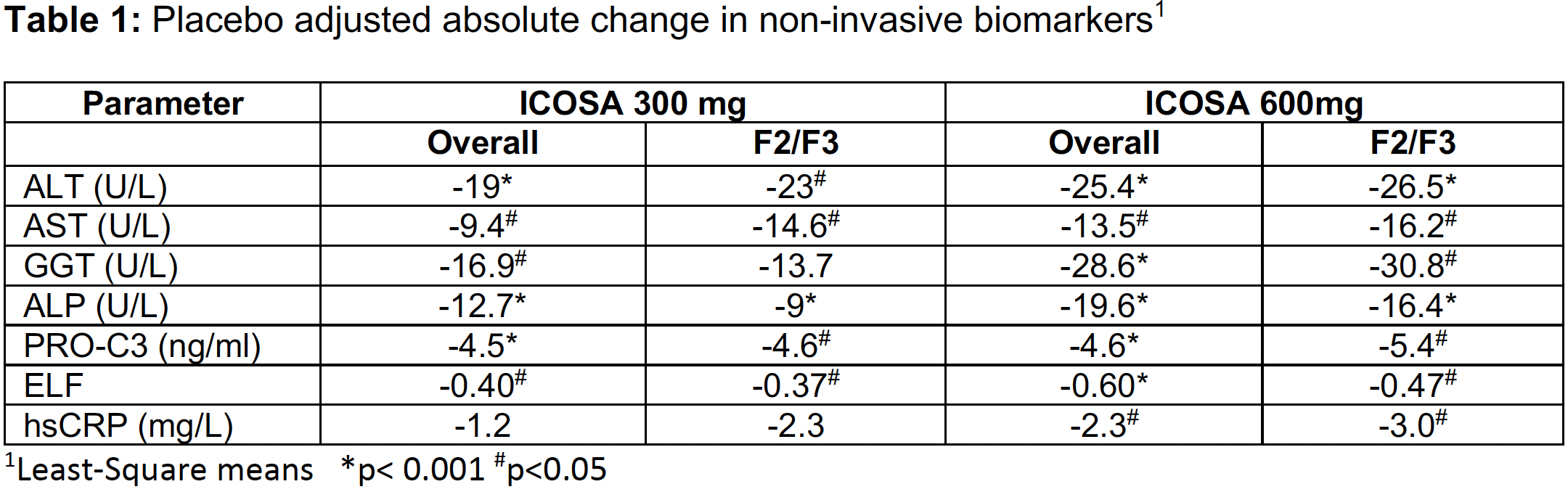
Importantly, patients with elevated markers of inflammation and fibrosis at baseline (ALT >60 U/L, PRO-C3 >15.5 ng/ml and hsCRP >3.0 mg/L) had a significantly greater treatment effect on these parameters compared to all subjects.
Professor Stephen Harrison, acting Chief Medical Officer at Northsea Therapeutics said, “These data demonstrate the potential for icosabutate to have a strong treatment effect across a broad range of patients. Based on preclinical and clinical data generated to date, along with the favorable safety profile, icosabutate has the potential to be the backbone for either mono- or combination therapy in NASH.”
Rob de Ree, Chief Executive Officer at Northsea added, “Northsea is committed to developing novel therapies for patients with life-threatening liver diseases. We are truly grateful to all of the patients and family members who have participated in this important study as well as the investigators and study teams who collaborated with us. With the ICONA trial now fully enrolled, we look forward to seeing these results translating into improvements in liver histology after 52 weeks of treatment and sharing the final results of the ICONA trial in the first quarter of 2023.”
Clinical Poster Presentation
Title: Icosabutate, a novel oral free fatty acid 4 (FFAR4) agonist, significantly decreases biomarkers of NASH and fibrosis independent of disease severity (Abstract #1915)
Authors: Naim Alkhouri, Ann C. Moore, Anita Kohli, Rashmee Patil, Madhavi Rudraraju, Stephen Rossi, David Fraser, Carine Beysen, Stephen A. Harrison and the ICONA Study Investigators
Date: November 12-15, 2021
Session: NAFLD and NASH: Therapeutics – Pharmacologic and Other
Presentation Type: Poster Presentation
A full list of presentations can be found on The Liver Meeting Digital Experience™ 2021 website.
The presentations will also be made available on the Northsea Therapeutics website (www.northseatherapeutics.com/publications) after the data has been presented at the meeting.
-End-
About NASH
NASH is a chronic liver disease characterized by liver inflammation and fibrosis and represents a more advanced stage of non-alcoholic fatty liver disease (NAFLD). It is frequently found in association with obesity and type 2 diabetes and is driven by multiple factors, including the formation of toxic lipid species in the liver. An estimated 15–30% of the adult population in developed countries have NAFLD, 10-15% of whom may advance to NASH, representing at least ~15–30 million patients in the 6 major markets. Further disease progression leads to advanced liver fibrosis and cirrhosis with a high risk of liver failure, hepatocellular cancer and the need for liver transplantation.
About Icosabutate
Fatty-acids and their metabolites function as signalling molecules in a broad array of pathways in the liver regulating metabolic, inflammatory and fibrotic responses. Icosabutate is an orally administered, structurally engineered, liver-targeted eicosapentaenoic acid derivative. The structural modifications result in high hepatic concentrations of non-esterfied icosabutate that in turn optimise targeting of fatty acid receptors of key relevance to NASH, including FFAR4. Preclinical data have demonstrated its therapeutic potential for treating fibrosing NASH and, of relevance to NASH patients, significant improvements in atherogenic lipids, glycemic control and inflammation have been shown in clinical studies. The potential to target both NASH and its associated comorbidities coupled with a favorable safety profile and oral administration, support icosabutate as a potential novel treatment for a broad range of patients with NASH.
About NorthSea Therapeutics
NorthSea Therapeutics B.V.(NST) is a Dutch biotech company focused on developing structurally engineered fatty acids (‘SEFAs’) for the treatment of NASH and other metabolic disorders. NST licensed the rights to its lead compound icosabutate and a library of SEFAs from Pronova BioPharma Norge AS, who developed LovazaÒ (US brand, branded OmacorÒ in Europe), a blockbuster cardiovascular drug. Icosabutate has been found safe and effective in two prior phase 2 clinical studies for treatment of hypertriglyceridemia and mixed dyslipidemia and is currently in clinical development for NASH. The icosabutate phase 2b ICONA NASH trial is scheduled to readout in the first quarter of 2023. Two additional SEFAs are in clinical development; SEFA 1024 is in phase 1 to be developed for dyslipidemia and SEFA 6179, to be developed for the orphan indication IFALD,
(Intestinal Failure Associated Liver Diease) will enter phase 1 in Q4-2021. NST is headquartered in the Netherlands with a presence in Norway and the US and is supported by Forbion Capital, Novo Seeds, BGV, NSV, venBio Partners and Sofinnova investments. Find out more about us online at: www.northseatherapeutics.com
For further information:
NorthSea Therapeutics B.V.
Rob de Ree (CEO)
E-mail: rob.deree@northseatherapeutics.com
Tel: +31 35 699 3000
Instinctif Partners (Media)
Melanie Toyne-Sewell / Katie Duffell / Grace Rutter
E-mail: NorthSea@instinctif.com
Tel: +44 20 7457 2020
