Study results identify NASH patients with Type 2 Diabetes as the key target population for Icosabutate therapy
− ICONA study assessed the safety, tolerability and efficacy of Icosabutate, a novel, dual FFAR1/FFAR4 (GPR40/GPR120) agonist in NASH patients with F1-F3 fibrosis.
− An increase in the proportion of patients achieving NASH resolution without worsening in fibrosis and a ≥2-point decrease in NAS was seen in the 600mg Icosabutate treated arm vs placebo (25.8% vs.11.9%, p=0.03), although the less stringent primary endpoint (not including NAS score) did not reach statistical significance in the overall study population.
− Significant treatment effect in diabetic NASH: In NASH patients with Type 2 diabetes (T2D), 600 mg Icosabutate significantly increased the proportion of patients achieving:
– NASH resolution without worsening of fibrosis (35.5% vs. 8.7% placebo, p=0.02)
– NASH resolution without worsening of fibrosis and ≥2-point decrease in NAS (35.5% vs. 4.3% placebo, p=0.007)
– ≥1-stage fibrosis improvement without worsening of NASH (19.4% vs. 0% placebo, p=0.02)
− Using AI technology, (qFibrosis, Histoindex) a 31% (p=0.03) treatment effect size was observed for regression of fibrosis stage in T2D patients treated with Icosabutate 600 mg.
− NorthSea will pursue a registrational development path for Icosabutate for the treatment of NASH patients with T2D.
– NorthSea to advance two additional clinical Phase 2 SEFA programs, i.e. SEFA-1024 for severe hypertriglyceridemia (SHTG) and SEFA-6179 for the orphan indication IFALD (Intestinal Failure Associated Liver Disease).
Amsterdam, The Netherlands, November 10, 2023 — NorthSea Therapeutics B.V. (NST), a biotech company developing novel and innovative strategies for the treatment of non-alcoholic steatohepatitis (NASH) and other metabolic diseases, today announced that the results of the ICONA phase 2b study for the treatment of NASH, will be presented as an oral late-breaker at the AASLD Liver Meeting in Boston on November 13, 2023.
The abstract, published today, describes that although the primary endpoint did not reach statistical significance, a significant increase in the proportion of patients achieving a more stringent endpoint, NASH resolution without worsening in fibrosis and a ≥2-point decrease in NAS, was seen in the 600 mg Icosabutate treated arm versus placebo (25.8% vs. 11.9%, p=0.03). A significant treatment effect size (31.2% placebo adjusted, p=0.007) was observed for the same endpoint in T2D patients.
Based on the highly favorable response (both histological and non-invasive biomarkers) to therapy in T2D patients, NorthSea will pursue a registrational development path for Icosabutate in NASH patients with T2D.
Professor Stephen Harrison, MD, consulting CMO, commented:
“The high prevalence of NASH in patients with diabetes is well established. What is less well known is that subjects with both diabetes and NASH have a high prevalence of underlying significant liver fibrosis. Thus, there is a clear need for safe, well tolerated and efficacious therapies in this patient population that target both inflammation and fibrosis. Icosabutate’s mechanism of action, targeting FFAR1 and FFAR4, which regulate insulin secretion, insulin resistance and hepatic inflammation, provide biological rationale for the observed effects in diabetic patients. Overall, these study findings are highly promising with respect to the potential for Icosabutate as a backbone oral therapy for diabetic NASH.”
Details of the ICONA Phase 2b data
Two hundred and eighty patients were randomized in the ICONA trial, of which 178 per protocol patients met the histologic criteria (F1-3, NAS ≥ 4, ballooning ≥1, inflammation ≥1) based on a 3- member independent panel read. Patients were randomized 1:1:1 to receive once-daily, oral Icosabutate 300mg, Icosabutate 600mg or placebo for 52 weeks.
The primary objective was to establish the proportion of patients with NASH resolution without worsening of fibrosis. Secondary objectives evaluated fibrosis improvement, as well as changes in markers of liver injury, inflammation, glycemic control, atherogenic lipids and safety and tolerability of Icosabutate. A subgroup analysis was performed in patients with T2D which comprised ~50% of the total population.
The largest treatment effect was observed in patients with T2D, with 35.5% (p=0.007 vs. placebo) of T2D patients treated with Icosabutate 600 mg achieving NASH resolution and ≥2 point decrease in NAS compared to 4% in placebo-treated patients. For fibrosis improvement without worsening in NASH, 28.6% (p=0.005) and 19.4% (p=0.02) of T2D patients achieved a ≥1-stage improvement in the 300mg and 600mg arms respectively, versus none in placebo. A dose response in fibrosis stage regression (qFibrosis) was observed using AI technology (Histoindex) with a treatment effect size (placebo adjusted) in T2D patients of 13.7% (p=0.32) and 31% (p=0.03) in the 300mg and 600mg treatment arms respectively.
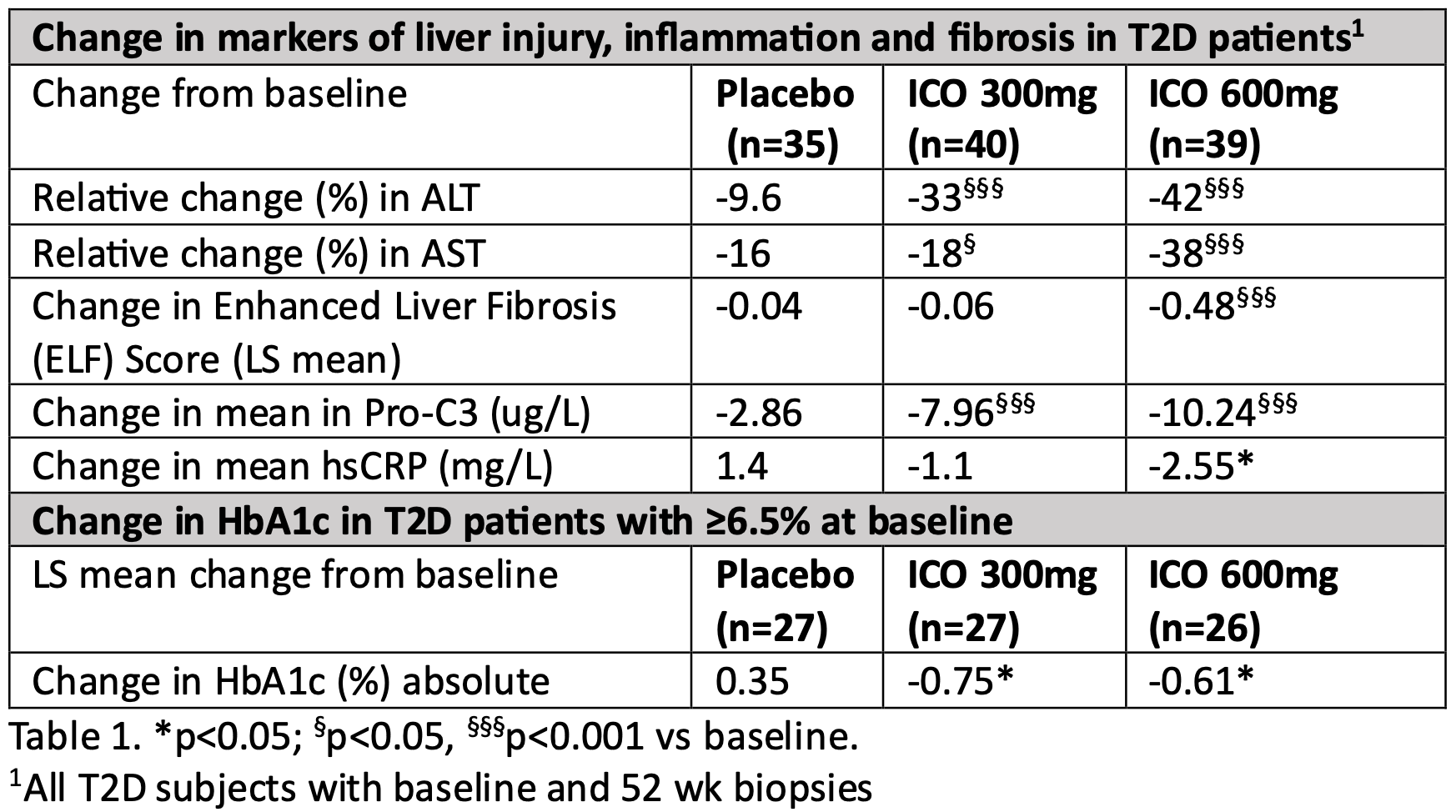
Icosabutate markedly improved all markers of liver injury, inflammation, fibrosis, glycemic control (placebo corrected ~1% decrease in HbA1c in T2D patients with ≥6.5% at baseline), atherogenic lipids and hsCRP in a dose-dependent manner (Table 1). Both doses of Icosabutate were well tolerated.
Arun Sanyal, Interim-Chair of the Division of Gastroenterology, Hepatology and Nutrition at VCU/VCU Health, added: “As is implied by the novel nomenclature, MASH (metabolic dysfunction associated steatohepatitis), metabolic dysfunction is an upstream driver of liver disease. The ability of Icosabutate to improve multiple markers of metabolic dysfunction, including insulin resistance, glycemic control, systemic inflammation and oxidative stress, are thus aligned with the improvements in histology and could potentially meet a key unmet need in NASH patients with type 2 diabetes”.
Rob de Ree, CEO at NorthSea Therapeutics, said: “The ICONA trial is the first time that patients with NASH have been treated with Icosabutate. The significant improvements shown across both biomarker and histology parameters for patients with type 2 diabetes is important and, in our view, holds great promise. We believe that this data allows us to make a strong argument in favour of further clinical development of Icosabutate in Type 2 diabetes. The findings also validate the potential of targeting FFAR as a therapeutic approach for the treatment of diabetic NASH, either as monotherapy or in combination with other drugs in clinical development. We look forward to advancing Icosabutate as a safe, oral therapy for the treatment of diabetic NASH. In the meantime, we are pushing forward with the development of our other SEFA programmes.”
-End-
Notes to Editors
About NorthSea Therapeutics
NorthSea Therapeutics B.V.(NST) is a Dutch biotech company focused on developing structurally engineered fatty acids (‘SEFAs’) for the treatment of NASH and other metabolic disorders.NST licensed the rights to its lead compound icosabutate and a library of SEFAs from Pronova BioPharma Norge AS, who developed Lovaza® (US brand, branded Omacor® in Europe), a blockbuster cardiometabolic drug. Icosabutate has been found safe and well tolerated in two prior phase 2 clinical studies for treatment of hypertriglyceridemia and mixed dyslipidemia and is currently in clinical development for NASH.
Two additional SEFAs are in clinical development: SEFA-1024 has been developed for SHTG and now has entered Phase 2a; SEFA-6179, which completed a phase 1 study in Q4-2022, is beingdeveloped for the orphan indication IFALD (Intestinal Failure Associated Liver Disease).NST is headquartered in the Netherlands with a presence in Norway and the US and is supported by Ysios Capital, Forbion Growth, Forbion Ventures, Novo Holdings, BGV, NSV, venBio Partners andSofinnova investments. Find out more about us online at: www.northseatherapeutics.com.
SEFA-1024
SEFA-1024 is an oral, highly lipophilic SEFA for the treatment of severe hypertriglyceridemia that is designed to target the gut-liver axis and to concurrently improve plasma lipids and glycemic control. NST has concluded a Phase 1 clinical trial with single and multiple ascending doses and a drug-to- drug interaction study of SEFA-1024 in healthy volunteers with an excellent safety profile and encouraging early signs of improvements in atherogenic lipids and lipoproteins. A Phase 2 proof-of- concept study started in H2 2023.
SEFA-6179
NST is also developing SEFA-6179 – a novel, oral, fully synthetic medium chain fatty acidanalogue – for the treatment of intestinal failure-associated liver disease, (IFALD), an orphan liver disease affecting individuals on prolonged parenteral nutrition. The unique pharmacologic properties of SEFA-6179 facilitate intestinal uptake, which is critical for ensuring optimal drug absorptionin patients with intestinal failure and short bowel syndrome and has demonstrated strong anti- inflammatory and anti-cholestatic effect in pre-clinical parenteral nutrition induced liver injury models. A phase 1 single and multiple ascending dose study has been successfullycompleted. SEFA-6179 has advanced into Phase 2 in H2 2023.
For further information:
NorthSea Therapeutics B.V.
Rob de Ree (CEO)
E-mail: info@northseatherapeutics.com
Tel: +31 35 699 3000
Instinctif Partners (Media)
Melanie Toyne-Sewell / Giulia Lasagni / Adam Loudon
E-mail: NorthSea@instinctif.com
Tel: +44 20 7457 2020
